Professor James Economy's Group
|
|
[Activated Carbon Fibers] [Ion Exchange Fibers] [Chelating Fibers] [Membranes] [Porous Inorganic Fibers]
Activated
Inorganic Fibers Templated from ACF In recent
years, inorganic materials have attracted much attention
as effective adsorbents for air and water treatment. Some
of them have specific adsorption ability to some gases,
such as MgO and CaO for high-temperature
sequestration of CO2 1, while MgO
and ZnO for H2S 2. CaO, MgO, CoO
and ZnO dispersed activated carbons display antibacterial
activity for B. subtilis and S. aureus 3.
Inorganic ion exchangers (e.g. M2Ti2O3SiO4.nH2O
(M = H, Na), crystalline silicotitanates) would allow
highly selective removal of the trace nuclear
contaminants such as Cs+ and Sr 2+
from water 4. TiO2 has been
used as a photocatalyst for water treatment 5;
and Ag as an antibacterial for E.coli 6.
Templating
is a technique for the preparation of porous materials
that has received widespread attention in the literature7.
The preparation of silica-based mesoporous materials by
using surfactant aggregates as structure directing agents
is a successful example of this synthetic strategy 8.
An
inorganic adsorbent fiber would be a valuable material
because it could have much higher contact efficiency and
thermal/oxidative stability allowing it to be regenerated
at higher temperatures in air. A number
of new inorganic fibers have been prepared in our group
by a templating approach where the ACF is coated with the
desired metal oxide precursor and then the carbon burned
out using air to yield a high surface area oxide fiber
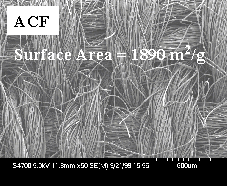
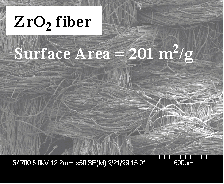
with the reverse image of the ACF. Fibers of ZrO2,
TiO2, MgO, CaO Al2O3 and
ZnO have been prepared in this manner and it is expected
that many of these materials will find use as catalyst
support which others could be used for sequestering CO2
at elevated temperatures.
Figure 1.
Cartoon for the conversion from ACF to Inoranic fibers
Figure 2. ACF
fabric to ZrO2 fabric References 1.
Kyaw, K., Kanamori, M., Matsuda, H., Hasatani, M. (1996).
Study of carbonation reactions of Ca-Mg oxides for high
temperature energy storage and heat transformation. Journal
of Chemical Engineering of Japan. 29(1), 112-18. 2.
Squires, A.M., Graff, R.A. and Pell, M. (1971).
Desulfurization of fuels with calcined dolomite. I.
Introduction and first kinetic results. Chemical
Engineering Progress, Symposium Series, 67(1115),
23-34. 3.
Tamai, H., Katsu, N., Ono, K. and Yasuda, H.
(2001). Antibacterial activated carbons prepared from
pitch containing organometallics. Carbon. 39(13),
1963-1969. 4.
Solbr, S., Allison, N., Waite, S., Mikhalovsky,
S.V., Bortun, A. I., Bortun, L.N. and Clearfield, A.
(2001). Cesium and Strontium Ion Exchange on the
Framework Titanium Silicate M2Ti2O3SiO4.nH2O (M = H, Na).
Environmental Science and Technology. 35(3),
626-629. 5.
Legrini, O., Oliveros, E. and Braun, A. M. (1993).
Photochemical processes for water treatment. Chemical
Reviews (Washington, DC, United States). 93(2),
671-98. 6.
Cowlishaw, J., Spadaro, J. A. and Becker, R.O. (1982).
Inhibition of enzyme induction in E. coli by anodic
silver. Journal of Bioelectricity. 1(3),
295-304. 7.
Benak, K., (2001). Synthesis and characterization of
novel adsorbent fibers, Ph.D. thesis, University
of Illinois at Urbana-Champaign, 8.
Kresge, C. T., Leonowicz, M. E., Roth, W. J.,
Vartuli, J. C. and Beck, J. S. (1992). Ordered
mesoporous molecular sieves synthesized by a
liquid-crystal template mechanism. Nature
359(6397), 710-12. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||