Professor James Economy's Group
|
|
[Bactericides Materials] [HDD Lubricants] [New materials for Fuel Cell] [Gas Hydrates] Potential Application of Gas Hydrate for Sea Water Desalination Gas
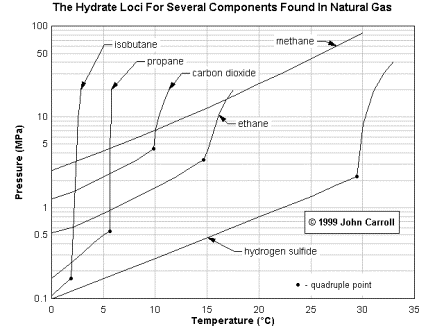
Hydrates are formed in systems of water and small
molecules by low temperature and high pressure. From a
scientist’s point of view, gas hydrates are cages of
water (host) molecules that surround gas (guest)
molecules and at the same time exclude the salt. Gas
hydrates are ice-like crystalline structures and can
exist at temperatures above the freezing point of water
and elevated pressures. It is estimated that there is
more methane in the form of hydrate sitting on the bottom
of the ocean there all of the oil ever discovered. In
this study, we propose, for the first time, to examine
the use of gas hydrates (clathrates) as a source of clean
water. We expect to be in a position to design and
evaluate a process for actually preparing potable water
from seawater. Gas
hydrates were formed in our laboratory using a high
pressure liquid propane or CO2 or at
lower pressure using ethane gas, with simulated sea water
at 1-4 oC. The initial results show that
gas hydrates display significant desalination, but the
salt concentration is still higher than the potable
level. Our
Research Plan is : 1). Develop an
understanding of the conditions required for gas hydrate
formation accompanied by salt separation; 2). Identify
approaches to catalyze the formation and decomposition of
gas hydrate; 3). Try to find out which of these
systems is appropriate for use at shallow depths for
economical recovery of potable water and the
working gas 4). Eventually, try to access the
methane hydrate sitting at depth of several thousand feet
to recover streams of clean water and methane ( a source
of fuel). http://www.agt.net/public/jcarroll/HYDR.HTM
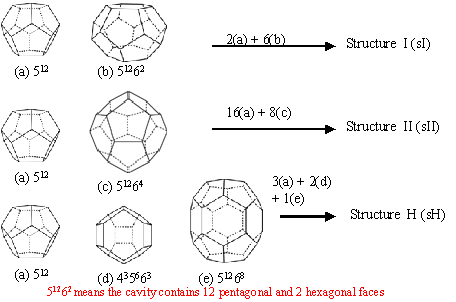
Figure 1.
Structures of gas hydrates Figure 3. Forming and Instant Melting of Ice-like Sample
References: 1.
Von Stackelbery, M and Müller, H. R. Z. f ür
Elektrochemie, 1954, 58, 25 2.
Ripmeester, J.A., et al., Nature, 1987, 325, 135 3.
E. D. Sloan, Jr, Geological, 1998, 137, 31-50
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||