Professor James Economy's Group
|
|
[Bactericides Materials] [HDD Lubricants] [New materials for Fuel Cell] [Gas Hydrates] New
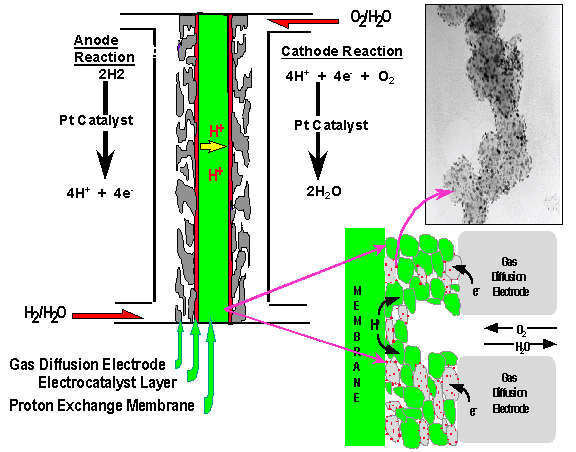
Materials For Fuel Cells Fuel
cells are electrochemical engines that produce
electricity from paired oxidation/reduction reactions
with flows of fuels and oxidant in and products out. Compared
with traditional thermal engines, fuel cells operate
quietly and efficiently, when hydrogen is used as fuel,
only power and drinking water generated.
Thermodynamically, they are not limited by the Carnot
efficiency. Hence, fuel cells can help reduce the
consumption of primary energy and emission of carbon
dioxide. The
automotive market is by far the largest potential market
for fuel cells where Proton Exchange Membrane Fuel Cell
receives the most attention. The membrane is usually a
perfluorosulfonic acid polymer, i.e. Nafion, developed by
Dupont in the 1960’s. There are mainly three
challenge of PEMFC used in the automobile needed to
improve: proton exchange membrane, electro catalyst and
hydrogen storage on board. Currently,
we focus on the development of alternative membranes for
Nafion. There are three goals we are trying to achieve:
synthesize membranes that are cheaper, lower methanol
crossover than Nafion and keeping high ion conduction
even at the temperature above 100oC, i.e.
under anhydrous environment. ( http://www.engin.umich.edu/dept/che/research/thompson/Plenary%20Lectures/Vanden%20Bussche.ppt
) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||