Professor James Economy's Group
|
|
[Activated Carbon Fibers] [Ion Exchange Fibers] [Chelating Fibers] [Membranes] [Porous Inorganic Fibers]
Novel
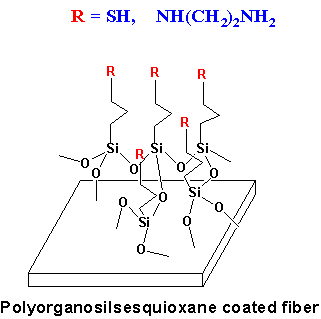
Organic-Inorganic Hybrid Chelating Materials The objective
of this project is to design novel tailored
organic-inorganic hybrid chelating materials for
environmental remediation, catalysis, and separations. Up
to now, we have prepared a series of these new kinds of
materials with tailored porosities in the form of fiber,
powder, or granule. The covalently bound organic
chelating groups in these materials include thiol, copper
(II) ferrocyanide, iminodiethanol, cyclodextrins,
calix[n]arenes, etc. These chelating materials are shown
to be extremely efficient in removal of trace organic and
inorganic contaminants, such as mercury, cesium, silver,
humic acid, p-nitrophenol, nitrogen dioxide, from
contaminated water or air. Our continuing studies on the
preparation and application of these chelating materials
are currently in progress. Key Features: • Permit removal of
trace contaminants in the presence of high concentrations
of Na+ and K+
Polyvinyl
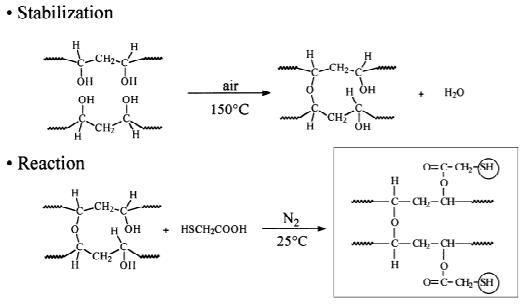
Alcohol Mercaptyl Fibers for Arsenite Chelation The work
described here entails the synthesis (Fig.1)and characterization
of polyvinyl alcohol mercaptyl fibers, coated on a
fiberglass substrate, for the purpose of removing
arsenite (As ) from water. Because thiols are chemically
the most active functional groups found in cells and are
capable of forming very stable complexes with metal ions,
this functional group was selected as an excellent
candidate for arsenite removal from water. The fibers
were characterized through infrared spectroscopy,
elemental analysis, analytical titration, scanning
electron microscopy, and environmental scanning
microscopy (Fig.2). The
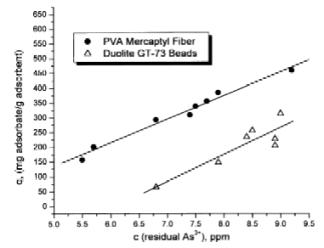
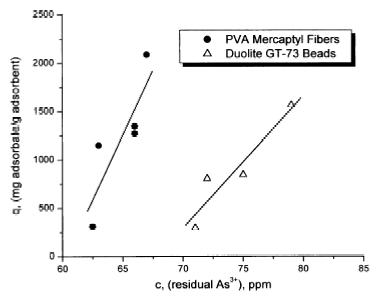
ability of these systems to chelate arsenite was measured
using equilibrium adsorption isotherms at initial
concentrations of 10 and 100 ppm (Figs 3 and 4). The ability
to regenerate these systems is also described. The
fibrous mercaptyl system’s performance is compared
to the commercial product, Duolite’s GT-73, a
macroreticular polystyrene–divinylbenzene resin with
chelating thiol functional groups. Fig. 1. Polyvinyl alcohol mercaptyl fiber synthesis.
Fig. 2. SEM. of PVA fiber functionalized at 50oC. Ref. L. Dominguez, Z.
Yue, J. Economy, and C. L. Mangun. “Design
of polyvinyl alcohol mercaptyl fibers for arsenite
chelation”. Reactive & Functional Polymers
53(2-3), 205-215. (2002) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||