Professor James Economy's Group
|
|
[Carbon/BN Composite] [AlB2 Composite] [Polymer Composites]
Boron
Nitride Composites For
this project, Dr.
Chris Mangun, President of EKOS Material Inc.
Printable Pdf file Several
years ago we discovered that we could convert borazine
oligomers, by heating at 1200°C, to a stable form (not
attacked by moisture) of BN in 90% yield. The oligomer is
derived from the self-condensation of borazine at 70°C,
also in about 90% yield. We found that using the borazine
oligomer we could prepare composites with PAN or pitch
based fibers that displayed excellent mechanical
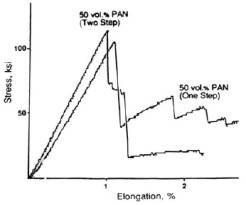
properties, see Figure 1. One should remember that
CVI is essential to produce a C/C composite with
acceptable mechanicals. We postulated and were
subsequently able to prove that the borazine oligomer
formed a liquid crystalline phase, which tended to match
the coefficient of thermal expansion of the fiber at the
interface. Surprisingly with the pitch-based carbon
fibers the composite displayed even on heating from
1200°C to 1500°C, see Figure 2.
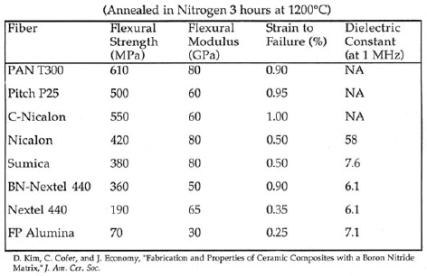
We
have successfully prepared BN matrix composites with SiC,
Al2O3 (both DuPont and Sumitomo
grades), and Nextel fibers, see Table 1. We have
also been able to infiltrate the borazine oligomer into
C/C structures with densities up to 1.3 g/cc to obtain
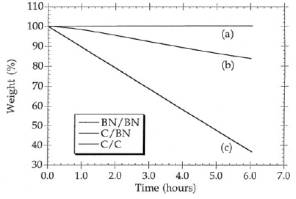
C/C/BN composites. The oxidative and hydrolytic stability
of the C/BN and C/C/BN composites is greatly improved
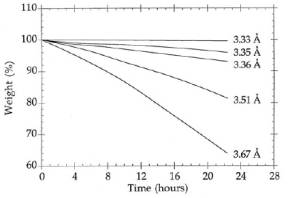
over the C/C composites, see Figure 3 and Figure
4. Previous researchers also demonstrated that the
hydrolytic and oxidative stability of Boron nitride is
dependent on the 002, or d-spacing, of the material. The
dependence is shown in Figure 4.
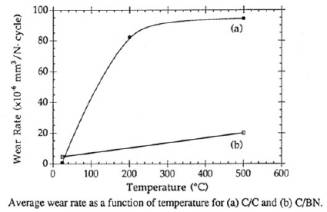
Using
high temperature pin on disk wear measurements we
observed a five-fold improvement in wear resistance of
C/BN over C/C, see Figure 5. More recently we have
installed an inertial brake dynamometer, which provides
friction and wear testing more suitable for the aircraft
braking industry. Preliminary data confirms the excellent
wear resistance of the C/C/BN as well as indicating that
the wear film is far more stable than that formed in our
test of C/C composites.
Potential
Applications of Borazine Derived Boron Nitride Matrix
Material For Brakes Presently,
aircraft brakes are usually manufactured out of
carbon/carbon, an expensive and relatively mechanically
weak ceramic-matrix composite. The use of such an
unwieldy and difficult material is necessary because of
the extreme temperatures and loads seen by the brakes
during landing cycles and rejected takeoffs. However,
despite its high temperature strength, carbon/carbon is
limited because carbon oxidizes at as low as 500°C. A
typical aircraft landing cycle may heat a plane's brakes
up to 800°C or higher. Hence, despite the use of
oxidation barriers, oxidation occurs in aircraft brakes
and weakens the material, increasing the wear rate and
reducing the useful lifetime of the brake. Boron
nitride has a higher oxidation temperature than the
carbon matrices that make up present aircraft brakes,
allowing the BN brake to retain more of its properties
during an aircraft landing or rejected takeoff. In
recent, members of our group have shown that the
oxidation resistance of BN is dependent on the d-spacing
of the material. The use of borazine as a polymeric
precursor can lead to very small (and hence advantageous)
d-spacings, partially due to the recently discovered
liquid crystalline nature of borazine under certain
conditions. The
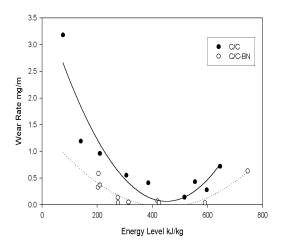
C/C-BN composites with densities of 1.55g/cc displayed
wear characteristics up to 50% lower than values observed
with C/C samples with densities of approximately
1.75-1.8g/cc. These observations held over the
entire energy spectrum typical of aircraft braking.
In addition, the wear rates at low energy (taxiing
conditions) and high wear rates (aggressive braking) were
significantly reduced (see Figure 6). This
behavior is attributed in part to the improved oxidation
resistance of the BN and its ability to facilitate
formation of a stable wear film.
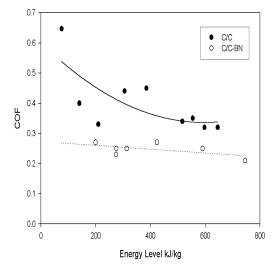
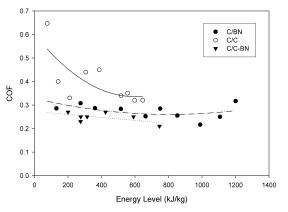
The
COF (see Figure 7), while being slightly lower
than the values for C/C, appeared much less sensitive to
changes in energy level. The significant decrease
in wear rate at 300kJ/kg, and ensuing increase at
600kJ/kg, produced no subsequent change in the COF.
This data, while preliminary, is extremely encouraging,
especially considering the less than optimum nature of
the samples. The
presence of the BN in the wear film (Figure 8)
imparted increased stability at all energy levels. Further
investigation of the low energy level wear film is
required to understand the role of the BN under those
conditions, although it appears the existence of a wear
debris film on the surfaces could lead to reduced wear.
The increased stability at high energy level is
attributed to the improved oxidation resistance of the
BN. BN does not display as low a COF as carbon but
as a braking material appears to have lowered both the
wear rate and COF in the low energy level regime. The
C/C-BN composites could be prepared to an average density
of 1.55g/cc via a liquid infiltration technique using a
borazine oligomer. Difficulties with processing led
to non-uniformed densities throughout the composites
where the surface density was as high as 1.6g/cc. These
difficulties are attributed to the less than ideal pore
structure produced from the original charring of the
phenolic resin. Improved processing techniques are
now being developed to produce BN containing composites,
which not only have a high density, but are uniform
throughout the cross-section. The
d002 spacing was larger than desired. Further
modification of the processing parameters is required to
yield lower interlayer spacings, which will increase high
energy level (temperature) performance of the composites
by increasing their resistance to attack by water. The
fabrication and wear results of higher density carbon
fiber/boron nitride composites (C/BN) and of C/C-BN
reinforced with a 3-D weave of carbon fibers present the
mechanism of wear at different energy levels.
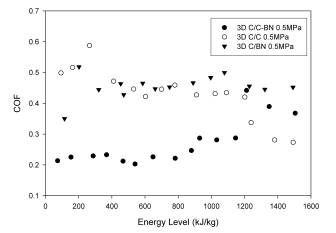
The 3-D needled reinforcement in the C/C system, coupled with the deposition of the matrix via CVI, yielded a material with greatly increased wear resistance. Wear rate dependence on energy appeared to remain unchanged, with wear rates higher at low energy levels and eventually increasing at the onset of oxidation. The changes in reinforcement and matrix appeared to have a modest effect on COF. The COF still varied from approximately 0.5 at low energy levels to approximately 0.25 at the onset of oxidation. (see Figure 9). In the case of the 3D C/BN, little effect was observed in the wear rate behavior with the addition of the 3-D needled carbon fiber, implying the wear rate is dominated by the matrix material in the case of the composites with a complete matrix of BN (see Figure 10). The one improvement noted was a shift in the energy level at which oxidation begins. It is believed increased thermal conductivity in the thickness direction, provided by the 3-D weave accounts for this increase. The 3-D reinforcement did have the effect of significantly increasing the COF. With the exception of the initial data point at low energy level the 3D C/BN displayed a value of approximately 0.45. As with all the composites containing BN the COF was found to be relatively insensitive to the energy level.
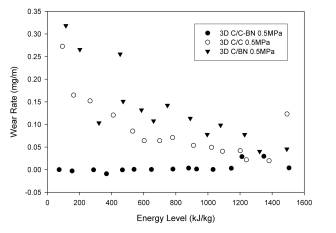
The
best results were obtained for the 3D C/C-BN, which
displayed little or no wear across the entire energy
spectrum tested. Based on the previously reported
data for C/C-BN the excellent wear resistance of these
composites was expected in the medium (low wear) energy
level regime. The outstanding wear resistance at
high energy level is attributed to the improved oxidation
resistance of the BN and the increased thermal
conductivity of the 3-D reinforced composites ( see Figure
11). In the low energy level regime the BN
facilitates the formation of the film-type wear debris
immediately, allowing extremely low wear rates. Interestingly,
the greatest wear resistance is realized only when the
hybrid matrix is used. The 3D C/C-BN also displays
a COF that is relatively constant throughout the normal
use range (up to 1000 kJ/kg) and then increases with
increasing energy level thereafter. Thus if one
imagines that 3D C/C-BN was used as an aircraft brake it
would perform consistently, displaying nearly constant
wear rates and COF, from taxiing conditions up to and
including RTO. Thin
Films and Adhesives A
second phase of our research involves creating
high-temperature adhesives and dielectric thin films from
BN derived from borazine. BN has promise as a material
for dielectrics in microelectronics because it has a very
high thermal conductivity and yet a low dielectric
constant. Earlier work in this group measured the
dielectric constants in the lateral and transverse
directions of a thin film of boron nitride derived from
borazine. However, presently, in order to stabilize the
borazine into boron nitride, a high-temperature (1200°C)
heat treatment is required. This is realistically too
high for microelectronics applications, because substrate
materials like Si and GaAs cannot be processed at such
temperatures. However, the liquid crystalline behavior of
the borazine may lead to alignment of the borazine when
in a thin film configuration, which could allow the
borazine to be stabilized at a much lower temperature.
Current research hopes to show that good-quality thin
films of boron nitride can be processed at temperatures
compatible with present-day microelectronics packaging. It
was noticed that the liquid crystalline behavior of the
borazine also has the effect of "matching" the
coefficient of thermal expansion of the reinforcement
material in a borazine-derived BN-matrix composite. Such
behavior may prove be useful if the borazine can be used
as an adhesive. This thermal expansion
"matching" behavior could lead to an adhesive
that could successfully be used to bond dissimilar
materials. The adhesive, being a ceramic, would be stable
at high temperatures. Such a high temperature adhesive
could prove useful in countless applications. Related
Publications 1.
D-p. Kim, C. G. Cofer, J. Economy, "Fabrication
and Properties of Ceramic Composites with a Boron Nitride
Matrix", Journal of the American Ceramics
Society 78, 1546-1552 (1995) 2.
D-p. Kim, J. Economy, "Fabrication of Oxidation
Resistant Carbon fiber / Boron Nitride Matrix
Composites", Chemical Materials 5,
1216-1220 (1993) 3.
C. G. Cofer, J. Economy, "Oxidative and
Hydrolytic Stability of Boron Nitride - A New Approach to
Improving the Oxidation Resistance of Carbonaceous
Structures", Carbon 33, 389-395 4.
D-p. Kim, J. Economy, "Occurrence of Liquid
Crystallinity in a Borazine Polymer", Chemical
Materials 6, 395-400 (1994) 5.
C. G. Cofer, et al., "Characterization of Fiber /
Matrix Interfaces in Composites with Boron Nitride
Matrix", Composites Science and Technology
50, 967 (1996) 6.
C. G. Cofer, A. W. Saak, J. Economy, "Carbon/Boron
Nitride Composites: An Alternative to Carbon/Carbon",
Ceramic Engineering and Science Proceedings 16,
663-671 (1995) 7.
C. G. Cofer, J. Economy, "Inorganic Polymer
Liquid Crystals", Polymer Liquid Crystal
Series, vol. 3, Chapter 3. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||