Professor James Economy's Group
|
|
[Activated Carbon Fibers] [Ion Exchange Fibers] [Chelating Fibers] [Membranes] [Porous Inorganic Fibers] Activated
Carbon Fibers
Typically,
activated carbon granules (ACGs) are used as adsorbents
to purify polluted waste streams because of their low
cost. However, the ACGs suffer from a number of drawbacks
including poor selectivity, slow kinetics, expensive
containment systems, less than 100 % working capacity,
and the need for a costly high temperature reactivation.
Also, there is a general lack of fundamental
understanding of the ACG product which sharply limits the
development of a predictive capability. In
the following section, to address some of these
disadvantages, we describe a number of innovations which
our group has devised to further improve the selectivity
and cost-effectiveness of the activated carbon system.
Starting
in 1970 while at the Carborundum Co. J. Economy
developed and commercialized for the first time an
activated carbon fiber (ACF) using a cross-linked
phenolic fiber (Kynol) as precursor. The Kynol fiber had
been commercialized in 1969 as a flame resistant
fiber. The fiber form of the ACF afforded greatly
improved contact efficiency, much higher capacity and the
potential for a high degree of design flexibility. In
1971, a joint venture was established by Carborundum with
Mitsubishi Chemical and Kanebo in Japan to manufacture
and commercialize the Kynol and ACF. Both fibers have
been available commercially from Nippon Kynol Inc since
that time. Subsequently, the Carborundum Co. was
acquired by Kennecott in 1977 and ceased any
activity in this area. Economy left in 1975 to join
IBM research where he focused on new materials for
advanced microelectronic devices. Control of Micropore Chemistry In 1989,
Economy joined the University of Illinois (Urbana) as
head of Materials Science and Engineering. Early
on, he undertook to reexamine the ACF’s
commercialized two decades earlier; namely, the high cost
of the ACFs (~ $100/lb), their relative fragility, and
the potential to control the pore surface chemistry.
Research in this last area progressed rapidly and it was
found that the selectivity could be greatly improved by
chemically converting the pore surface into acidic
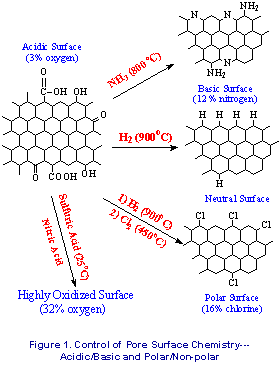
or basic and neutral or polar surfaces (Fig.1). The
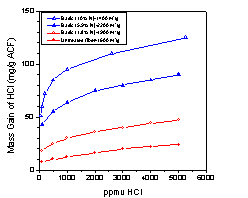
nitrided system (basic) displayed sharply increased
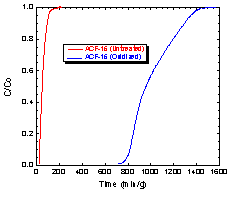
capacities for HCl gas (Fig 2), while the oxidized
system (32% oxygen and rich in carboxylic acids)
displayed a 20 X improvement in breakthrough times over
untreated ACF’s for ammonia removal. (Fig.3).
Starting
in the early 1990’s a program was initiated to
establish the validity of the widely held view that
the pores in activated carbon were slit shaped. As part
of this program we also wanted to determine how pores
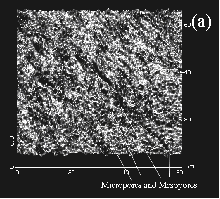
begin to form in the ACFs. Applying scanning tunneling
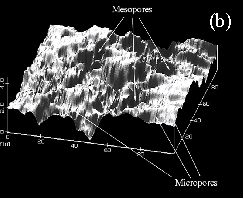
microscopy (STM) to a number of different ACF’s we
were able to access both the surface of the fiber
as well as the cross-section. The fiber surface in the
commercial product was highly etched displaying a
relatively high concentration of mesopores with varying
diameters from 20-100 Å. Examination of the fiber
cross-section showed a high degree of uniformity in the
dimensions of the micropores (10-18 Å) depending on the
degree of activation. (Fig.4) This uniformity
extended to within 100 Å of the fiber surface where the
larger pore at the etched surface necked down to the more
uniform porosity in the core. From this study there was
no evidence for slit shaped pore even after an
examination of over 800 STM’s taken from the
cores and surfaces of a number of different ACF’s. Figure. 4. Scanning Tunneling Microscopy (STM) of (a) a cross-section and (b) a highly etched surface of activated carbon fiber (ACF) An
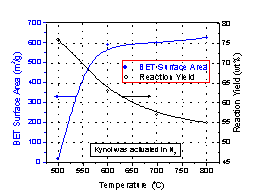
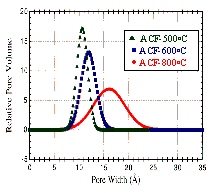
insight into the origins of the microporosity was
afforded by an examination of a phenolic fiber heated to
600oC in nitrogen. It was found that a
micropore structure (8-10Å) was formed with a surface
area of 600 m2/g. Hence the presence of a
stable cross-linked structure which persists during
heating to 600oC (Fig.5) (weight loss 30%) was
sufficient to preserve the microstructure (Fig.6). Further
heating up to 800oC using an etchant such as
CO2/ H2O led to much higher surface
areas and introduction of several percent oxygen at the
surfaces of the micropores.
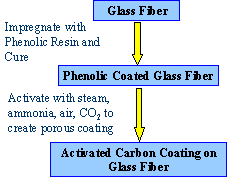
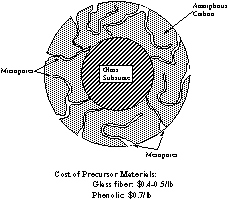
New Invention: ACF on Fiberglass A major
advance concerning cost and fiber durability was
made in the mid 1990’s with the discovery that the
process for preparation of the ACFs, could be greatly
simplified using a fiber glass substrate coated with
30-70 % phenolic resin (Figs. 7 and 8). For example, with
fiber glass paper ($0.4-0.5/ lb) and a phenolic resin
precursor ($0.7/ lb). The cost of ACF’s could be
greatly reduced from over $100/lb to values approaching
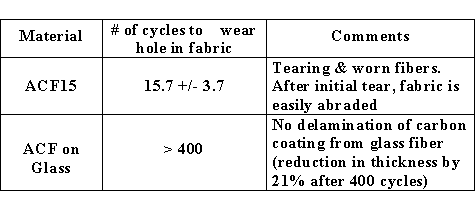
those of GAC. In addition, the wear
resistance was improved dramatically by 30 X over the
ACF’s (see Table below). Presumably the
intrinsic strength of the glass fiber core was protected
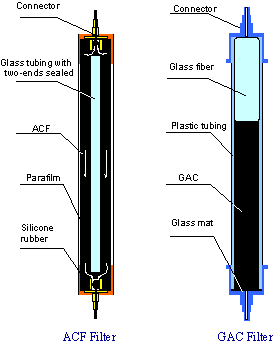
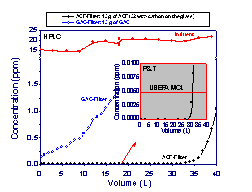
by the surface coating of activated carbon. As can be
seen in Figs 9 and 10, a comparison of cartridges
consisting of similar amount of granular activated
carbon and ACF on glass paper showed an enormous
improvement in removal of trace contaminants such
as benzene to levels below 1 ppb. The GAC filter
which is designed for use in removal of benzene was
ineffective in removing benzene down to the maximum
contaminant level of 5 ppb ( a value designated by
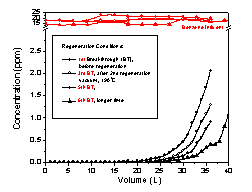
USEPA). It is noteworthy that the cartridge based on ACF
could be regenerated repeatedly to its original
activity by heating to190oC for 2 hrs under
vacuum (Fig.11). Typically regeneration of GAC’s is
carried out by heating to 800oC which leads to
an increase in pore diameter. This requires a further
step involving chemical vapor deposition of carbon to
recover the original pore diameter. As a result, the
GAC’s are often disposed of in land field. Areas
of opportunity where our work has demonstrated clear
advantages include: 1) the recovery of ammonia and amine
compounds from industrial waste streams, 2) adsorption of
trichloroethylene (TCE) which is air stripped from
wastewater streams, 3) adsorption of herbicides from
groundwater, 4) removal of CO2 from
closed-loop anesthesia systems, 5) adsorption of
hazardous pollutants from air for use in respirators and
chemically protective clothing, and 6) the adsorption of
SO2 from flue gas at coal fired plants.
Figure 9. Design of ACF and GAC Filters
In the
last several years , we have found that we can use
a catalyst to convert a number of polymers to high
surface area fibers at relatively low temperatures. This
method which is referred to as chemical activation is
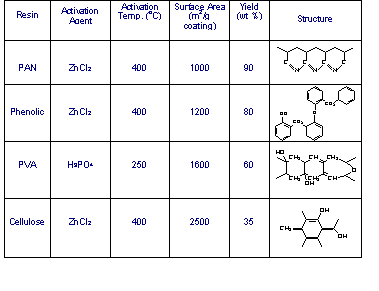
carried out by coating a glass fiber with polymers
such as phenolic, polyacrylonitrile (PAN), polyvinyl
alcohol (PVA), and cellulose along with a catalyst such
as H3PO4, ZnCl2, or
NaOH. High surface areas from 1000-2500 m2/g
are achieved by heating from 250-450oC. As
opposed to ACF’s where yield of 5-25% are obtained
during activation much higher yield can be obtained by
chemical activation, for example, 90% (PAN), 80%
(phenolic), 60 % (PVA) and 35% (Cellulose). Some unusual
pore surface chemistry can be obtained, for example with
PAN we form a polyquinizarine structure (19-20% N) which
has significant basic character. In the case of PVA a
significant amount of oxygen persists as hydroxyl groups
which imparts a strong hydrophilic character to the
surface of the micro/mesopores. Presumably, with
suitable selection of the precursor polymer, we can
change the chemistry of the micropore surface without
having to resort to the high temperature treatment
described earlier. The polyquinizarine coated glass fiber
also appears to exhibit potential for air pollution
control, namely for sequestering carbon dioxide. Table 2. ACF
Made with Chemical Activation 1.
Lin, R.Y.; Economy, J., "The Preparation and
Properties Of Activated Carbon Fibers Derived From
Phenolic Precursor", Appl. Polym. Symp. 21,
143-152, 2.
Economy, J.; Lin, R.Y., "Adsorption Characteristics
Of Activated Carbon Fibers", Appl. Polym. Symp.
29, 199-211,1976, 3.
J. Economy. “Now that's an interesting way to make a
fiber!” CHEMTECH 10(4), 240-7. 1980. 4.
Mangun, C.L.; Daley, M.A.; Economy, J. "Chemical
Modification of Activated Carbon for Enhanced Removal of
Toxic Contaminants," Air & Waste Management
Association, 88th Annual Meeting, San Antonio, TX,
June, 1995. 5.
J. Economy, M. A. Daley, E. J. Hippo, and D. Tandon.
“Elucidating the pore structure of activated carbon
fibers through direct imaging using scanning tunneling
microscopy (STM)”. Carbon 33(3), 344-5. 1995.
6.
Daley, M.; Tandon D.; Economy, J.; Hippo, E.J.
"Elucidating the Pore Structure of Activated Carbon
Fibers through Direct Imaging using Scanning Tunneling
Microscopy (STM)," Carbon, 34 (10),
1191-1200,1996. 7.
Daley, M.A.; Mangun, C.L.; Economy, J. "Low-Cost
Activated Carbon Fiber Assemblies for Control of
Contaminants from Air/Water," Air & Waste
Management Association, 89th Annual Meeting,
Nashville, TN, June, 1996. 8.
Daley, M.A., C.L. Mangun and J. Economy, "Predicting
adsorption properties for ACFs," Preprints of
Div. of Fuel Chem., 41(1), 326-330 1996. 9.
J. Economy, M. Daley, and C.L. Mangun. “Activated
carbon fibers - past, present, and future”.
Preprints of Papers - American Chemical Society, Division
of Fuel Chemistry 41(1), 321-5. 1996. 10.
Daley, M.A.; Mangun, C.L.; DeBarr, J.A.; Riha, S.;
Lizzio, A.A.; Donnals, G.L.; Economy, J. "Adsorption
of SO2 onto Oxidized and Heat Treated Activated Carbon
Fibers (ACF's)," Carbon, 35(3), 411-417, 11.
Mangun, C.L.; Daley, M.A.; Braatz, R.D.; Economy, J.
"Effect of Pore Size on Adsorption of Hydrocarbons
in Phenolic-Based Activated Carbon Fibers," Carbon,
36 (1-2), 123-131,1998. 12.
J. Economy and C. L. Mangun. “Design of a high
efficiency, low cost activated carbon fiber system for
removal of trace contaminants”. Preprints of
Symposia - American Chemical Society, Division of Fuel
Chemistry 43(4), 880-884. 1998. 13.
Mangun, C.L.; Braatz, R.; Hall, A.; Economy, J.
"Fixed Bed Adsorption of Acetone and Ammonia onto
Oxidized Activated Carbon Fibers," Industrial
& Engineering Chemistry Research, 38 (9), pgs.
3499-3504, 14.
Economy, J., C.L. Mangun, “Design of ACF for
chemically protective masks/clothing,” Proc. of
the 1998 ERDEC Scientific Conference on Chemical and
Biological Defense Research, 365-371, 1999. 15.
Mangun, C.L., K.R. Benak, M.A. Daley, J. Economy, “Oxidation
of activated carbon fibers: effect on pore size, surface
chemistry, and adsorption properties,” Chemistry
of Materials, 11(12), 3476-3483, 1999. 16.
Economy, J., C. Mangun, “Design of new materials for
environmental control,” Macromolecular Symposia,
143, 75-80, 1999. 17.
Benak, K., L. Dominguez, J. Economy, C. Mangun, Z. Yue,
“Control of Organic/Inorganic contaminants utilizing
tailored ACFs,” Proc. of the Annual AWWA
Conference, Denver, CO, 2000. 18.
Z. Yue, C. L. Mangun, J. Economy, P. Kemme, D. Cropek,
and S. Maloney. “Removal of chemical contaminants
from water to below USEPA MCL using fiber glass supported
activated carbon filters”. Environmental Science
and Technology 35(13), 2844-2848. 2001. 19.
Mangun, C.L., J.A. DeBarr, J. Economy, “Adsorption
of sulfur dioxide on ammonia-treated activated carbon
fibers,” Carbon, 39(11), 1689-1696, 2001. 20.
Mangun, C.L., K.R. Benak, J. Economy, K.L. Foster, “Surface
chemistry, pore sizes and adsorption properties of
activated carbon fibers and precursors treated with
ammonia,” Carbon, 39(12), 1809-1820, 2001. 21.
J. Economy, L. Dominguez, and C. L. Mangun. “Polymeric
ion-exchange fibers”. Industrial &
Engineering Chemistry Research 41(25), 6436-6442. 2002.
22.
Economy, J., C. Mangun, “Novel fibrous systems for
contaminant removal,” In Sampling and Sample
Preparation for Field and Laboratory, Ed. J.
Pawliszyn, Elsevier Science 2002. 23.
Z. Yue, C. L. Mangun, and J. Economy. “Preparation
of fibrous porous materials by chemical activation. 1.
ZnCl2 activation of polymer-coated fibers”.
Carbon 40(8), 1181-1191. 2002. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||